Biomedical Engineering brings together engineers and clinicians with the aim of improving clinical tools by the application of engineering research, techniques and innovation to the field of clinical medicine.
Engineers and clinicians are united in their ethos of striving to achieve the best practical outcomes in their fields founded on high-quality scientific research and innovation.
Philip Guildford, Director of Research
Biomedical Engineering here at the University of Cambridge unites numerous research groups across the departments and faculties through multidisciplinary networks and collaborative research initiatives, delivering medical technology innovation that impacts fields of unmet healthcare need. The increase in Biomedical research here at the Department, has been in part due to the generous support of the Evelyn Trust along with funding from a private donor, and from the Newton Trust.
The Evelyn Trust Lectureship in Engineering for Clinical Practice was established in 2008 and Dr Graham Treece has held the post since then working at the interface between engineering and medicine and encouraging others into this active and rewarding field.
During this time there have been many inspiring projects that have brought together researchers from many different departments across the university. Here are just some of the exciting projects:
Bioengineering model of Eustachian tube dysfunction
The Eustachian tube is a narrow structure running between the air-filled middle ear space and the back of the nose. It is designed to allow ventilation of the middle ear, but in around 5% of the adult population, the tube fails to open adequately, causing many different types of ear disease. In children, Eustachian tube dysfunction is even more common, often causing glue ear and hearing loss. Until recently, medical staff concentrated on treating the consequences of the disease in the ear rather than the underlying defect with the Eustachian tube itself.

Balloon Eustachian Tuboplasty (BET) is a new minimally-invasive technique using a narrow balloon to stretch the Eustachian tube and has recently been used in patients. It appears to be effective at treating conditions caused by poor tube opening. There is much that needs to be understood about how the technique works. In a collaboration between Dr. Michael Sutcliffe here at the Department of Engineering, and Mr. James Tysome in the Ear Nose and Throat Department at Addenbrooke’s Hospital, they study the biomechanics of balloon inflation within human Eustachian tube tissue. In parallel, physical and 3D computer-based models are created to add to clinical observations, and provide methods of testing innovative adaptations to the procedure, and new equipment modifications.
The BET procedure: A balloon catheter is inserted into the Eustachian tube via the nose. It is then inflated to a high pressure for two minutes, before being deflated and removed.
Dr. Michael Sutcliffe Department of Engineering
Mr. James Tysome Consultant ENT Surgeon, Addenbrooke's Hospital
Dr. Matthew Smith Clinical Research Associate and Specialist Registrar in ENT Surgery, Addenbrooke's Hospital
Respiratory flows: improving understanding and diagnosis

The sounds that propagate to the chest have been used as a tool to diagnose disease in the chest since the time of Hippocrates, however this was made much more practical with the invention of the stethoscope by Rene Laënnec in 1819. Since then a great deal of work has been done to refine this technique, its advantage over more recent imaging methods being the fact that it is non-invasive, non-radioactive, and relatively cheap. The disadvantage is a lack of specificity. Wheezing (a particular type of unhealthy lung sound) alone has been linked to at least 20 different conditions, and so it is hard to make a specific diagnosis using sounds heard at the chest. The aim of our work is to make diagnosis using lung sounds more specific. We believe that the way to do this is to gain a better fundamental understanding of how sound is produced and propagates in the chest. Specific ways in which we plan to accomplish this include confirming a mechanism for the production of wheezes, pleural rub, and placing a sound source at a known location within the chest and measuring the response at the chest. By studying sound sources the team hope to be able to distinguish a larger number of distinct sounds, and the study of sound propagation will aid doctors in locating the source of any unhealthy sounds, which once again would help them to differentiate between currently similar noises. This work will be completed in collaboration with Professor Edwin Chilvers at Addenbrookes Hospital.
Dr. Anurag Agarwal Department of Engineering
Mr. Alastair Gregory Department of Engineering
Prof. Edwin Chilvers Professor of Respiratory Medicine, Department of Medicine
Cardiovascular acoustics

Heart diseases produce characteristic sound which can be heard at the chest with a stethoscope. This work attempts to understand the causes of these sounds and use this knowledge to create and smart stethoscope, capable of automatic diagnosis. One example of a sound producing valvular heart disease is aortic stenosis, which produces a crescendo-decrescendo sound as blood is pushed through the valve. We will attempt to understand the cause of this sound by testing artificial heart valves, modified to simulate aortic stenosis, in water. These results will be correlated against recordings from 60 patients, with varying degrees aortic stenosis, from Papworth Hospital. From these results, we hope to produce an algorithm capable of differentiating the sound signal of aortic stenosis from a healthy heart sound signal and also predict the severity of the stenosis.
Dr. Anurag Agarwal Department of Engineering
Mr. Edmund Kay Department of Engineering
Dr. Len Shapiro Consultant Cardiologist, Papworth Hospital
Organ transplants
The success of an organ transplant critically depends on the development and connection of new blood vessels to the implant. Before such a functional blood network is established, the supply of oxygen and nutrients to the implant are sparse. This environment can result in severe damage to the pre-mature blood vessel connections, causing detrimental effects to the implant viability. An emerging treatment is based on cell therapy, where engineered blood vessel cells with an enhanced fitness are injected into the patient, to replace the defective networks. However, due to the limitation in testing techniques, the functionality of these therapeutic cells are not well understood. This project aims to develop a user-friendly experimental tool to better characterise the engineered blood vessel cells. It is based on a microfluidic chip combined with extracellar matrices, which can simulate the evolving physiological environments, post-transplantation. The ultimate goal is to provide the clinicians with a simple testing platform, which will deliver important information in regards to the cell therapy dosage and efficacy, thus improving treatment outcome and patient safety.
Dr. Yan Yan Shery Huang Department of Engineering
Prof. Andrew Lever Department of Medicine
Treatment for chronic pain based on real-time fMRI brain connectivity neural feedback
Chronic pain is estimated to affect up to 20% of the population at some point in their lives. Unfortunately many drug treatments are either ineffective or have significant side effects, leaving many people with pain with little or no adequate treatment. This has stimulated the search for new, innovate ways of treating pain. Dr Ben Seymour, a Wellcome Clinical Fellow at the Computation and Biological Learning Lab, and Dr Dan Wheeler, Consultant Anaesthetist in the Pain Clinic at Addenbrookes Hospital, are hoping to develop a new method using functional brain imaging. They aim to apply engineering-based machine learning methods to ‘decode’ the early changes in brain activity that are thought to contribute to the maintenance of chronic back pain, and then feed back the abnormal activity so that subjects can learn to consciously control it. If this works, this could become a new avenue for treating patients with back pain.
Dr. Ben Seymour Department of Engineering
Dr. Dan Wheeler Consultant Anaesthetist, Pain Clinic
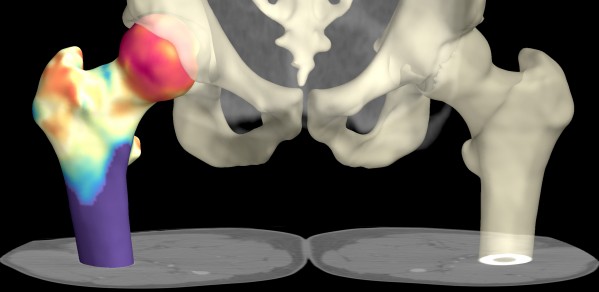
Cortical thickness estimation for osteoporotic hips
The distribution of cortical bone (the denser outer layer of bone) in the hip is believed to be critical in determining fracture resistance. Generally cortical bone around the femoral neck gets thinner with age, and can become as thin as an egg-shell (0.3 mm) by the eighth decade in critical areas. Current clinical practice is to measure overall hip bone mineral density (called a 'DXA' scan) which is not very sensitive to cortical thickness. Potentially, multi-detector computed tomography (MDCT) can be used to examine cortical thickness and 3-D structure directly. However, existing CT technology has a limited resolution, which is not good enough to be able to distinguish the bone cortex, particularly in clinically relevant thin cases.
A collaboration between the Department of Engineering and the Department of Medicine has led to the development of an algorithm which can accurately measure cortical thickness from clinical MDCT data and display this as a colour map over the bone surface. This has been demonstrated to be much more accurate than existing techniques for cortical assessment.
This new tool opens up many research possibilities. For instance a recent study on the treatment of bone with Teriparatide has already been able to demonstrate not only statistically significant bone growth in the 0.05 - 0.1 mm range, but also exactly where the bone growth is occurring. There are fascinating parallels between these areas and the stress distribution and muscle insertion points on the femur.
Dr. Andrew Gee Department of Engineering
Dr. Ken Poole Department of Medicine
Dr. Graham Treece Department of Engineering
The aim of the Engineering for Clinical Practice initiative is to provide:
- new and relevant research opportunities for staff in the Engineering Department
- new tools for clinicians at the Hospital, leading to better outcomes for patients
- income to both institutions from the commercial exploitation of research results
- involvement of staff from the Clinical School as investigators on new types of collaborative research projects
- enrichment of the engineering undergraduate course through the increased exploration of clinical applications

